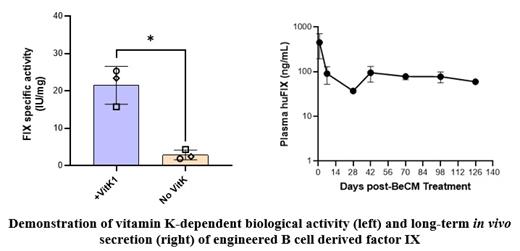
Hemophilia B is an X-linked recessive bleeding disorder that affects approximately 1:20,000 males. It is caused by mutations in the F9 gene that encodes for the factor IX (FIX) protein, an essential enzyme in the coagulation cascade. The current recommended therapy for hemophilia B patients is prophylactic administration of exogenous FIX derived from recombinant protein. The short biological half-life of FIX requires frequent infusions to maintain therapeutic level. More recently, an adeno-associated virus (AAV) vector-based gene therapy, etranacogene dezaparvovec, has been approved for some adults as a potential new option. Despite these advances, there remains a significant unmet medical need in hemophilia B. Intravenous infusions of weekly (or more frequently, 3 times per week) factor replacements are an enormous burden to patients and their caregivers. Gene therapy, while a promising option for some, carries potential for significant risk. Importantly, the limitations associated with increasing the dose of FIX needed to treat pediatric patients as they age make immunogenic AAV-based gene therapy inappropriate for this patient population. Terminally differentiated human plasma cells derived from genetically engineered B cells (termed B Cell Medicines, BeCMs), potentially offer natural longevity (persisting for decades), capacity for high levels of protein secretion (thousands of Ig molecules/cell/sec), the ability to engraft without host preconditioning, and the ability to re-dose, making them an attractive platform for the sustained supply of biologics where continuous dosing is required to achieve therapeutic benefit. We have developed an ex vivo precision engineered BeCM platform with modularity and broad therapeutic utility. In this study, primary human B cells were isolated, activated, and engineered by CRISPR/Cas9 genome editing followed by AAV-mediated homology directed repair (HDR) insertion of human F9 gene into the C-C chemokine receptor type 5 (CCR5) safe harbor locus. The cells were then further expanded and differentiated towards the plasma cell lineage, resulting in FIX-producing BeCMs. We achieved approximately 40% targeted integration as measured by droplet digital PCR (ddPCR). Engineered BeCMs secreted up to 60 ng/1e6 cells/hour of FIX protein, approaching 40% of IgG secretion rate as measured by ELISA. BeCM-produced FIX was analyzed by LC-MS which demonstrated gamma carobxylation of FIX protein Gal-domain. Vitamin K-dependent activated partial thromboplastin time (aPTT), using the one stage clotting assay, was employed to verify biological activity of BeCM-produced FIX. Similarly, FIX-expressing BeCMs exhibited vitamin K-dependent activity in vitro in the chromogenic assay. FIX-expressing BeCMs were transferred into immunodeficient NOG-hIL6 mice, with FIX production demonstrated at least 20 weeks in vivo. The safety of FIX-expressing BeCMs has been characterized based on 28-day and 5-month in vivo studies in NOG-hIL6 mice. Neither abnormal clinical observations nor mortality were observed in those studies. Biodistribution of the FIX expressing BeCM product was assessed using a qPCR assay. The qPCR data confirms the expected biodistribution of FIX-expressing BeCMs, which engraft and persist in bone marrow tissue over time. In summary, we have developed an ex vivo precision gene engineered B cell medicine that produces active and sustained levels of FIX for the treatment of hemophilia B. The potential therapeutic application of this unique biologic delivery system could afford a novel treatment modality for hemophilia B.
Disclosures
No relevant conflicts of interest to declare.